Pipeline
A robust pipeline in pursuit of transformative therapies.
Targeting the root cause of EBV-driven disease
We are working hard to address significant unmet need in cancer and autoimmune diseases with our robust pipeline of investigational T-cell immunotherapies, paving the way for future treatments.
Tabelecleucel (tab-cel®)
Tab-cel® is under investigation for patients with Epstein-Barr virus-associated post-transplant lymphoproliferative disease (EBV+ PTLD) and is in earlier stage development for other EBV-associated diseases.
LEARN MORE- Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- Tab-cel®, Atara's most clinically advanced T-cell immunotherapy in development, is currently being investigated in the Phase 3 registration-enabling ALLELE study to assess efficacy and safety for the treatment of EBV+ PTLD in SOT after failure of rituximab or rituximab and chemotherapy, and in HCT after failure of rituximab (NCT03394365, ALLELE study).
-
-
- Phase 2 multi-cohort study including EBV+ PTLD with CNS involvement, EBV+ PID/AID LPD, EBV+ LMS and other potential EBV-associated diseases (NCT04554914, 205 study).
ATA188
We're developing ATA188 as an investigational off-the-shelf, allogeneic T-cell immunotherapy that aims to specifically target EBV-infected B cells and plasma cells for progressive forms of multiple sclerosis (MS).
LEARN MORE- Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- ATA188 is currently in a Phase 2 EMBOLD clinical study for the treatment of patients with progressive forms of MS and has met target enrollment, with primary analysis and communication expected in November 2023.
Next-generation CAR T and AlloCAR T programs
Using a molecular toolkit of next-generation technologies, our platform can be modified to develop CAR T and AlloCAR T therapies to target a wide range of non-EBV-associated diseases.
ATA3219
Allogeneic CAR T targeting CD19, currently in clinical development, leverages our EBV T-cell platform and features a next-generation 1XX co-stimulatory domain.
LEARN MORE- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- Target: CD19
- Technologies: Novel CAR T 1XX co-stimulation
- Off-the-shelf, allogeneic
ATA2271/ATA3271
Autologous ATA2271 is in Phase 1 development and allogeneic ATA3271 is in preclinical development: both target solid tumors expressing the tumor antigen mesothelin and leverage novel next-generation 1XX co-stimulatory domain and PD-1 Dominant Negative Receptor technologies.
LEARN MORE- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- Mesothelin is expressed at high levels on the surface of cells in aggressive solid tumors including mesothelioma, triple-negative breast cancer, esophageal cancer, pancreatic cancer and non-small cell lung cancer
- MSK investigator-sponsored Phase 1 study (NCT04577326) of a mesothelin-targeted CAR T immunotherapy is ongoing
- ATA2271 Atara's CAR T collaboration with MSK will focus on development of a next-generation, mesothelin-targeted CAR T using novel 1XX CAR signaling and PD-1 dominant negative receptor (DNR) checkpoint inhibition technologies.
-
-
- Mesothelin is expressed at high levels on the surface of cells in aggressive solid tumors including mesothelioma, triple-negative breast cancer, esophageal cancer, pancreatic cancer and non-small cell lung cancer
Other next-generation AlloCAR T programs
We’re tackling the limitations and challenges of current CAR T therapies by developing novel next-generation allogeneic CAR T approaches.
- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- Targets: Multi-targeted CD19-CD20
- Technologies: Novel co-stimulation
-
-
- Targets: Undisclosed
- Technologies: Novel CAR T 1XX co-stimulation
- Partnered with Memorial Sloan Kettering Cancer Center
External Scientific Presentations
February 2023
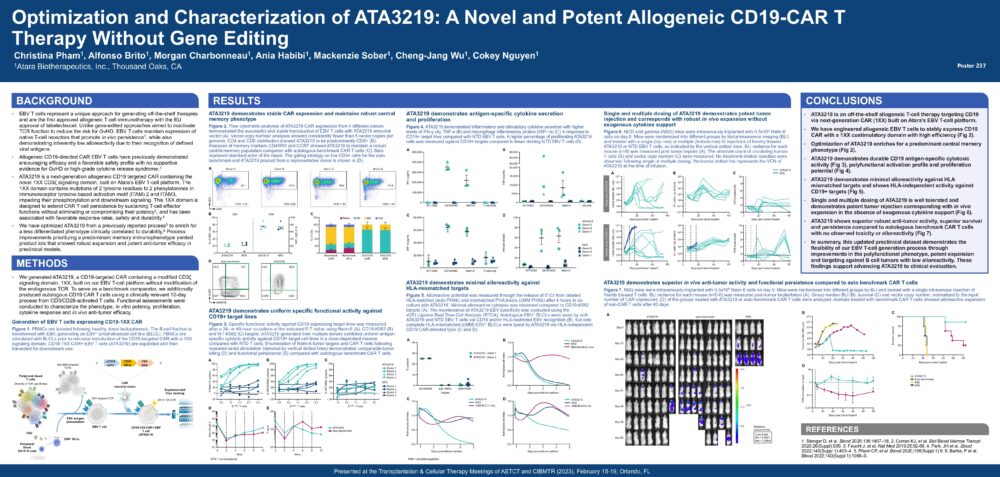
Optimization and Characterization of ATA3219: A Novel and Potent Allogeneic CD19-CAR T Therapy Without Gene Editing
Download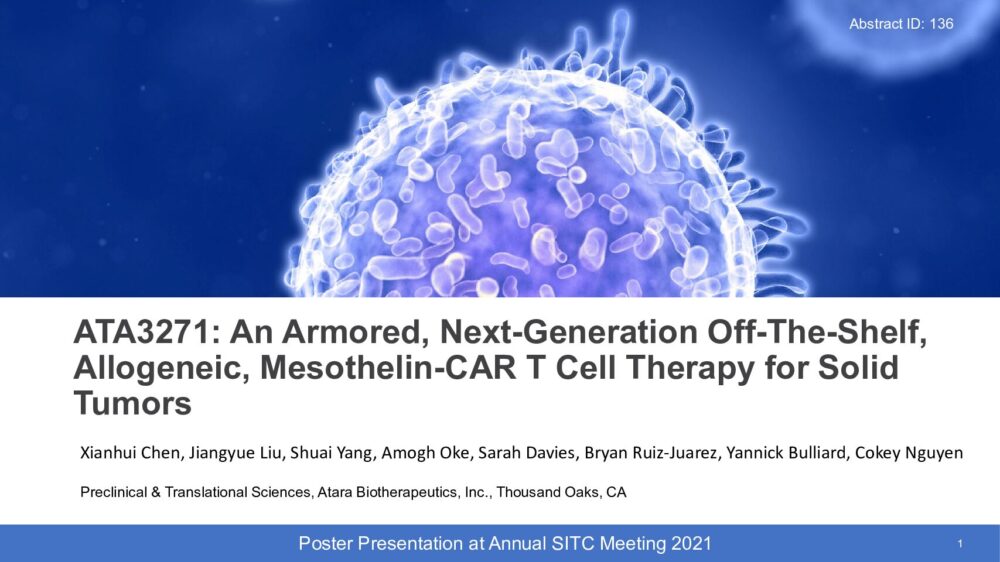
February 2022
ATA3271: An Armored, Next-Generation Off-The-Shelf, Allogeneic, Mesothelin-CAR T Cell Therapy for Solid Tumors
Download
November 2020
ATA3219: A Potent Next Generation Allogeneic Off the Shelf CD19 CAR T Therapy Without the Need for Gene Editing
Download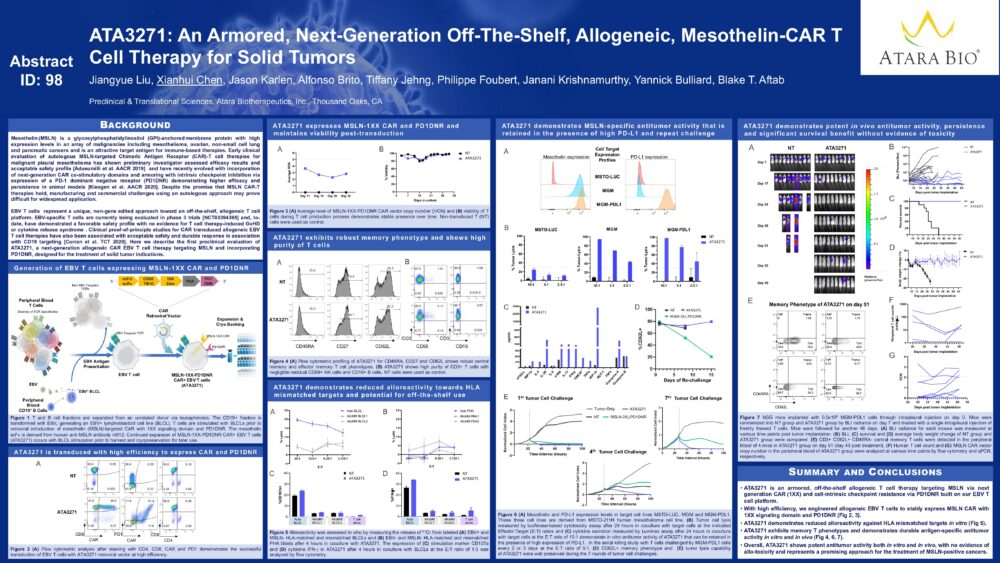
November 2020
ATA3271: An Armored, Next-Generation Off-The-Shelf, Allogeneic, Mesothelin-CAR T Cell Therapy for Solid Tumors
Download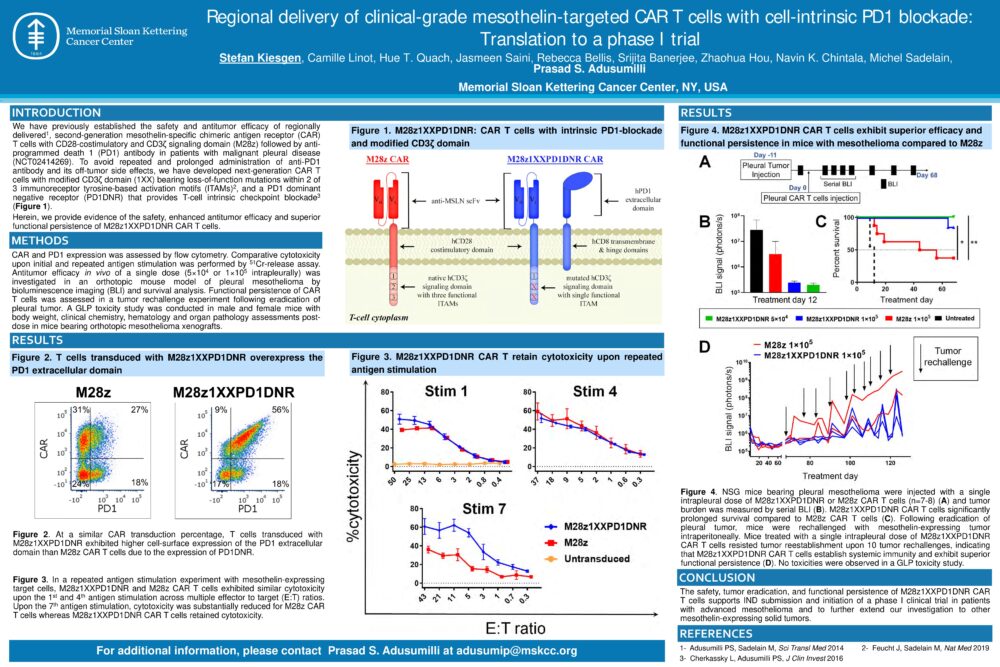
June 2020
Regional delivery of clinical-grade mesothelin-targeted CAR T cells with cell-intrinsic PD1 blockade: Translation to a phase I trial
Download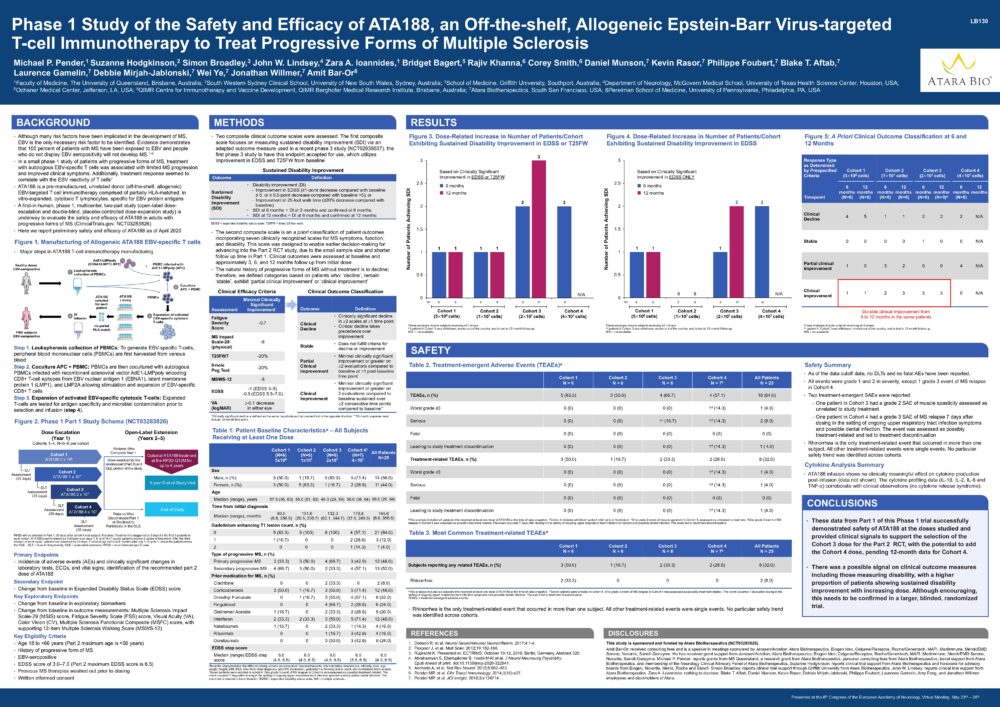
May 2020
Phase 1 Study of the Safety and Efficacy of ATA188, an Off-the-shelf, Allogeneic Epstein-Barr Virus-targeted T-cell Immunotherapy to Treat Progressive Forms of Multiple Sclerosis
Download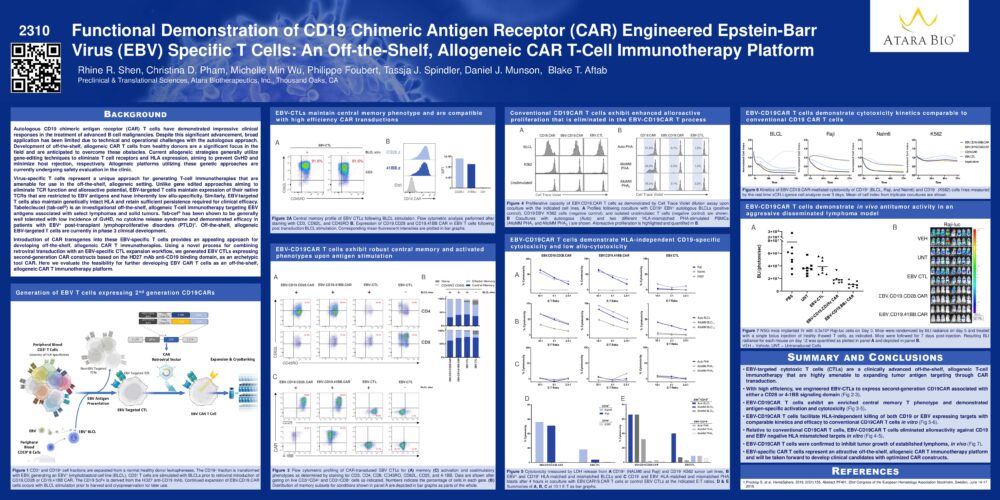
April 2019
Functional Demonstration of CD19 Chimeric Antigen Receptor (CAR) Engineered Epstein-Barr Virus (EBV) Specific T Cells: An Off-the-Shelf, Allogeneic CAR T-Cell Immunotherapy Platform
DownloadThese are investigational agents. Efficacy and safety have not been established.